Research
Each living organism, from bacteria to human, employs thousands to tens of thousands of different proteins to perform biological functions that sustain its survival and reproduction. While high-throughput approaches such as proteomics and DNA sequencing have been rapidly identifying new proteins, we remain at a bottleneck in finding out how all these proteins perform their biological functions. How can we solve this problem?
Rather than tackling the functional mechanism of each individual protein, we instead study the principles that govern the structures, dynamics, energetics and functions of proteins, to understand the core principles that are shared by a large families of proteins.
We have been studying the following fundamental problems in protein biology: (a) the mechanisms of receptor activation in biological signaling, (b) the principles of proton transfer in proteins, (c) the impact of the Hofmeister salts in protein structural dynamics, and (d) how heat shock protein 90 (HSP90) accommodates the vast structural diversity of client proteins.
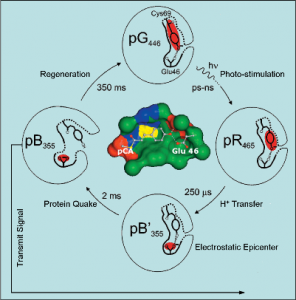 To illustrate one key insight into biological signaling, we discovered the “electrostatic epicenter” model of receptor protein activation. The “electrostatic epicenter” is generated through charge transfer, such as proton transfer, electron transfer or phosphorylation. The newly formed electrical charge is not stable, and subsequently drives a large amplitude protein quake that transforms a receptor protein from the receptive state to the signaling state, resulting in receptor activation. We predict that this model is applicable to a range of signaling proteins.
To illustrate one key insight into biological signaling, we discovered the “electrostatic epicenter” model of receptor protein activation. The “electrostatic epicenter” is generated through charge transfer, such as proton transfer, electron transfer or phosphorylation. The newly formed electrical charge is not stable, and subsequently drives a large amplitude protein quake that transforms a receptor protein from the receptive state to the signaling state, resulting in receptor activation. We predict that this model is applicable to a range of signaling proteins.
Developing new research tools empowers us to explore new territory in science and enable new discoveries. We are developing an emerging technology that we called infrared structural biology. A significant advantage of infrared structural biology over current methods is its capability to detect functionally important structural motions of a protein over a broad range of time, from picoseconds to seconds. In addition, infrared structural biology is highly sensitive to proton transfer and electron transfer during protein function, which are difficult to detect through X-ray crystallography.
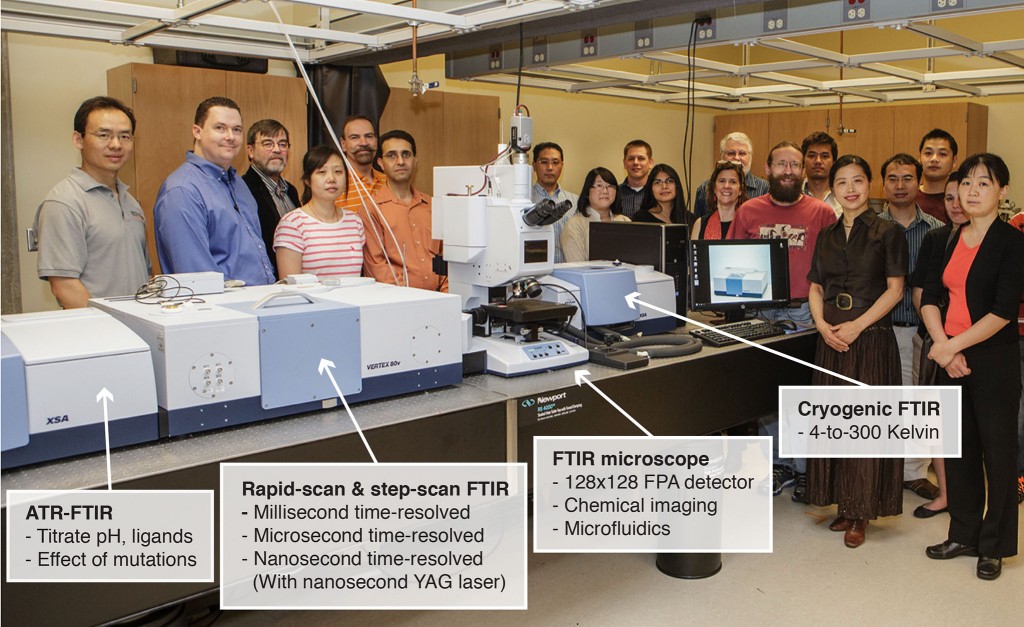
We have recently acquired and installed one of the best FT-IR systems worldwide, funded through an NSF MRI grant. Our state-of-the-art FT-IR systems offer 8 different advanced technologies, including microfluidic FT-IR, cryogenic FT-IR, nanosecond time-solved FT-IR, infrared chemical imaging with 16,000 liquid nitrogen cooled detecting elements, and an ATR FT-IR with dialysis to study drug binding and ligand binding to proteins.
The advanced FT-IR at the Henry Bellmon Research Center